Breath Analysis & Diabetes
Key takeaways:
- Diabetes begins with a condition called insulin resistance, which is caused by elevated free fatty acids and intramyocellular lipids.
- The accumulation of free fatty acids and intramyocellular fat results from impaired fat-burn efficiency by our cells and/or weight gain.
- Breath examination provides an excellent tool for tracking fat-burning efficiency and the likelihood of obesity.
Unfortunately, diabetes is as common as it is misunderstood. Although pre-diabetes and diabetes are among the most common conditions, many of us, including doctors and health professionals, are unsure of their origin. As a result, we may claim that diabetes is a lifestyle disease, but we just cannot put our fingers on what exactly in our lifestyle causes it. This article explains the biological process of diabetes and how breath analysis provides an excellent tool for predicting its onset.
What is diabetes?
Metabolism is the process by which our bodies transform the nutrients we eat into the energy we need to maintain essential functions (i.e., heartbeat, brain function, etc.), regulate temperature, and perform physical activities (e.g., move and exercise). Fat and carbohydrate are the two most commonly used nutrients in our metabolic process and thus supply more than 90% of our body's energy daily.
Although both macronutrients are used in significant amounts throughout the day, the process by which they are being treated varies vastly. This difference stems from the way they are stored. On the one hand, our body can store practically unlimited amounts of fat in adipose tissue (e.g., the fat accumulated around our abdomen, back, and other areas). Still, on the other hand, we can store only a minimal amount of carbohydrates. To put things into perspective, the average person may store up to 30,000 kcal worth of fat, which can increase tremendously by becoming overweight or obese, but can only store approximately 2,000 kcal of carbohydrates. The limited ability of our body to store carbohydrates ultimately means that whenever ingested, they need to be used immediately, stored in our small carbohydrate reserves (in case there is room), or converted into fat through a process called de-novo lipogenesis. Since the second pathway is unlikely and the third one energetically costly, our bodies will resort to the immediate burn of carbohydrates when ingesting them.
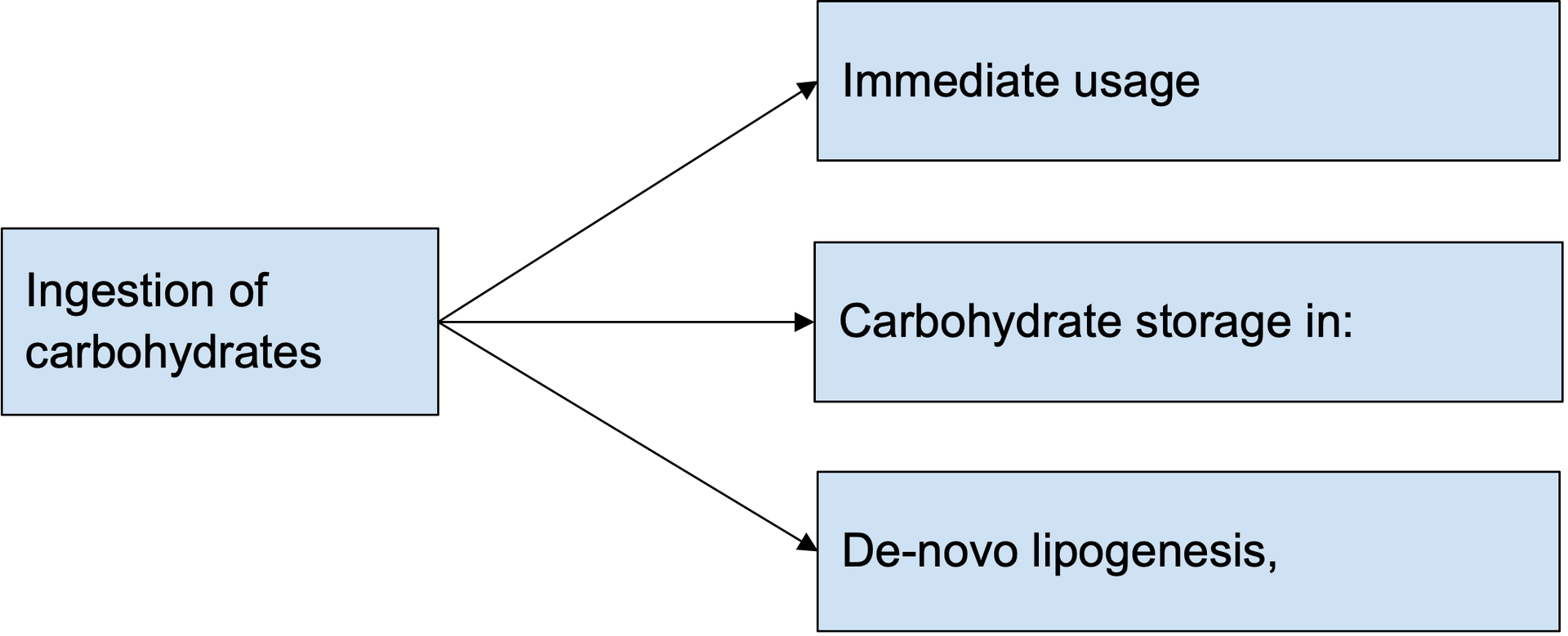
Let’s now look into the process by which our body burns carbohydrates. Much like fats, carbohydrates must reach the inner part of our cells to be processed by the mitochondria, our body’s energy factory that converts fats or carbohydrates into the calories we use to survive and move. To achieve this, the following process takes place. Initially, carbohydrates need to be converted into glucose, which begins in our mouth but primarily in our small intestine. The intestine membranes absorb glucose, enter the bloodstream, and transfer it to the liver, where it is used, stored, or directed to other body parts. When it enters our muscle mass to be stored for future use, it is converted into glycogen.
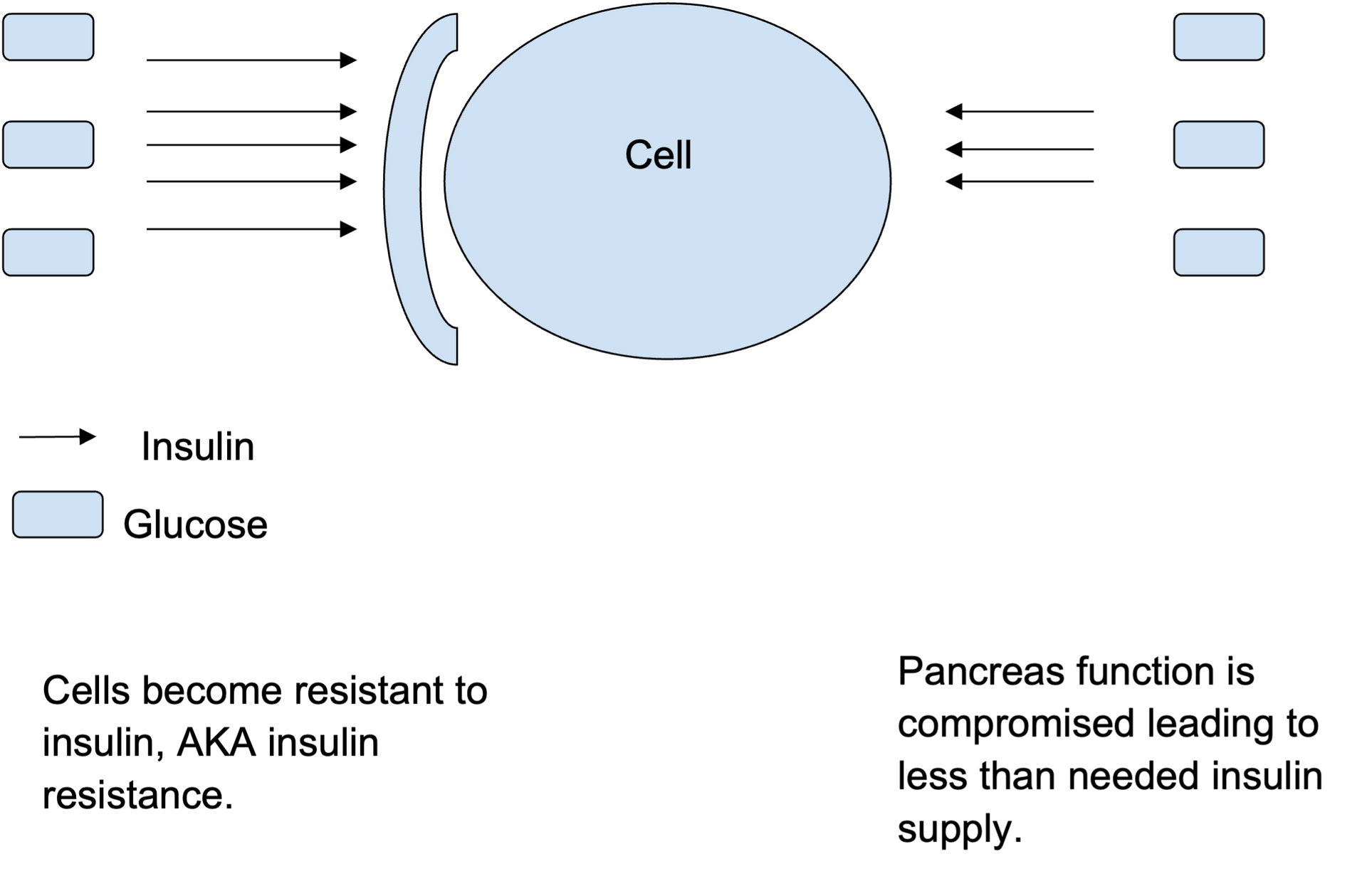
As glucose levels rise in the blood, our body reacts through the secretion of insulin, a hormone required for managing glucose across our organs. Specifically, insulin is a substance that “latches” onto glucose molecules and enables them to enter cells, thus clearing them from the bloodstream. This is necessary because although glucose is a valuable nutrient that provides useful energy, it can be toxic for our organs if it remains in the bloodstream for too long. The toxic effects of lingering glucose in the blood slowly degrade all forms of tissue and can cause heart disease, neurodegenerative diseases such as Alzheimer's disease, and, in advanced cases, even require amputation of one’s leg. As a result, once glucose enters the bloodstream from the membranes of our small intestine, it needs to be stored in the liver or used by the working muscles immediately. Our body uses the hormone insulin, secreted from our pancreas, to enable glucose to enter our liver and muscle cells. This is where the route of diabetes lies. Specifically, diabetes occurs if our pancreas doesn’t produce enough insulin or our cells are not responsive enough to it, a condition also known as insulin resistance
What causes diabetes?
As described above, diabetes is a confluence of two conditions: cells becoming unresponsive to insulin and the pancreas not producing enough insulin. The combined effect of the two phenomena is that insulin production cannot clear glucose from the bloodstream. The lingering glucose thus causes widespread deterioration of all tissues across our body. Although both conditions need to coexist for diabetes to occur, their onset is not simultaneous but sequential.
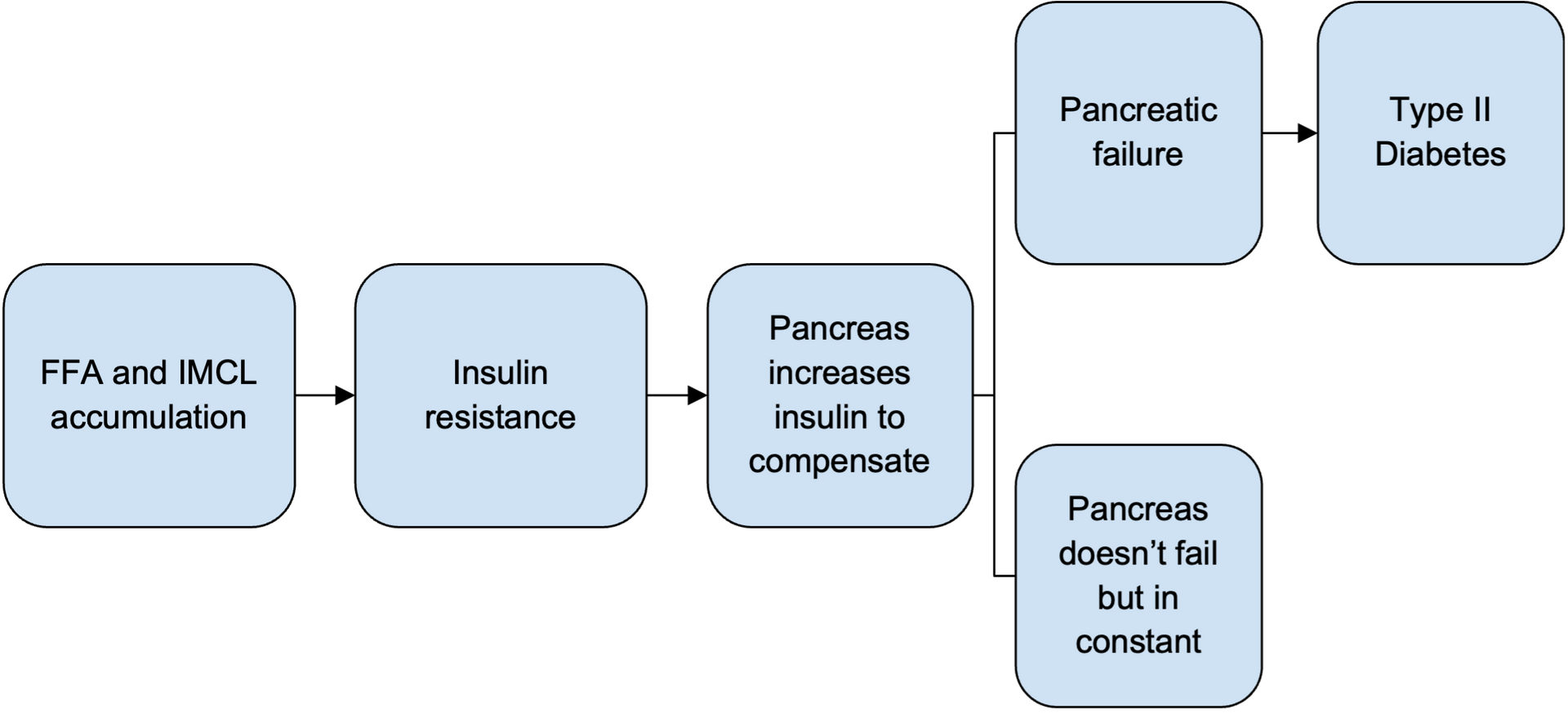
The first of the two conditions is insulin resistance caused by excessive accumulation of intramyocellular lipids (IMCL) and plasma-freefatty acids (FFAs). Intramyocellular lipids are fat stores within muscles, whereas free fatty acids are fat molecules circulating in our bloodstream. The common denominator between the two is that they cause cells to become less responsive to insulin and thus cause insulin resistance. These two conditions occur when overall fat accumulation across the body increases, or in other words, when one starts to transition from average weight to an obese or overweight state.
To compensate for the fact that cells are now less responsive to insulin, the pancreas of an individual with early stages of insulin resistance starts to secret more insulin. This sets the person’s pancreas in a constant state of “overdrive,” meaning it constantly operates above its normal capacity. It is important to note, however, that this doesn’t always lead to pancreatic failure and insulin secretion shutdown. Nearly 80% of obese and overweight individuals live in a state where their pancreas is secreting excessive insulin to compensate for their varying degrees of insulin resistance. This state is also known as hyperinsulinemia.
However, in case hyperinsulinemia leads to partial or complete pancreatic failure. Insulin levels drop sharply, circulating blood glucose cannot penetrate cells, and ultimately, lingering blood glucose starts to cause its deleterious effects. This is when the onset of Type II Diabetes (T2D).
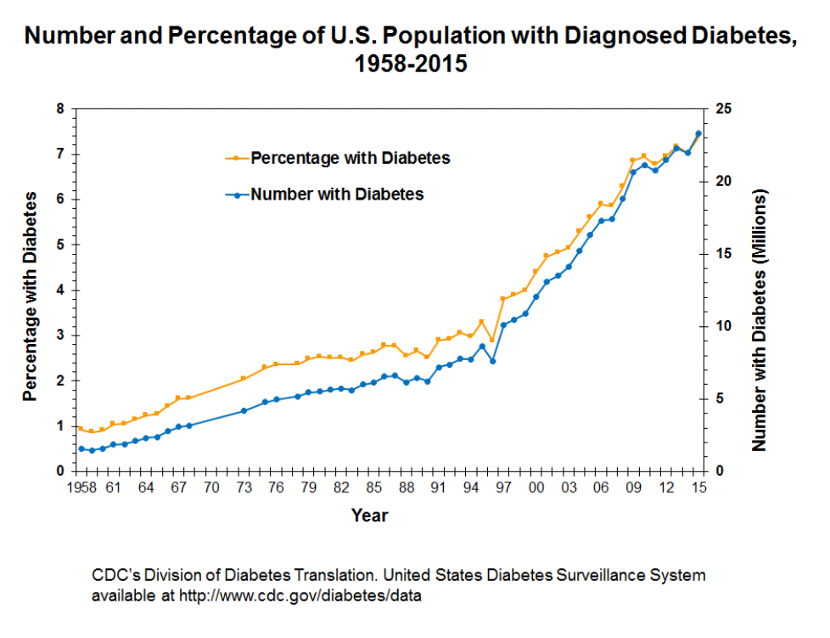
Given the above mechanism, it is evident that fat accumulation is the underlying driver of T2D. This is corroborated by every piece of longitudinal data about the disease and its correlation with obesity levels. Specifically, T2D started to become a health concern and subsequently a dire epidemic at the same time that people began gaining weight. Given that obesity is founded on our unhealthy nutrition habits and lack of physical activity, it is an undeniable fact that T2D is largely a disease of our modern lifestyle.
The myth of carbs causing diabetes and fat treating it
The above mechanism elucidates the nature of T2D and how it is routed in weight gain and fat accumulation. However, it also sheds light on the fact that the macronutrient composition of one’s diet is irrelevant. In other words, following a low carb high, fat or low-fat high, carb diet can’t cause or cure diabetes. Besides, we describe in another one of our blogs, “Obesity Explained,” that through millennia, humans have followed all kinds of diets ranging from almost exclusively fat and animal protein-based to almost solely carb-based ones. Despite the wide range of diets followed by our ancestors, diabetes never became a health concern until obesity came along. As a result, weight loss and re-ignition of the cells’ insulin sensitivity through physical exercise to insulin are the only ways to mitigate the effects of T2D or, in some cases, even cure it.
How breath analysis provides an early warning for diabetes
Diabetes begins when insulin resistance occurs due to toxins secreted by free fatty acids (FFAs) and intramyocellular lipid (IMCL) accumulation. These toxins affect the cells’ ability to respond to insulin and thus prevent them from being able to absorb glucose that is circulating in the blood. In simple words, insulin becomes less effective when insulin resistance occurs as the “key” for glucose to enter cells. Therefore, we must first understand the root cause of fat accumulation to uncover the origins of insulin resistance. Specifically, the build-up of FFAs and IMCL can be traced back to two factors:
- Reduced ability to burn fat: Fat is a fuel source that requires oxygen and higher mitochondrial density compared to carbohydrates. Simply put, it requires “well-training” mitochondria as it’s a more complex fuel to process. Lack of exercise or constant consumption of high glycemic index carbs will gradually reduce mitochondrial density, making your cells less able to burn fat as a fuel source. Less fat oxidation means higher intramyocellular lipids and free fatty acids in the blood.
- Obesity and visceral fat: Adipose tissue, in other words, accumulated body fat, impacts metabolism by releasing hormones and other substances, including leptin, cytokines, adiponectin, and proinflammatory substances. Another essential substance released in the process is FFAs. Individuals who are obese or overweight, therefore, have higher than average FFAs circulating in their blood. Out of the substances that adipose tissue releases that affect insulin sensitivity, the most are FFAs. The higher the level of circulating FFAs, the higher the insulin resistance and, thus, the likelihood of T2D.
Both phenomena can be traced or predicted through breath analysis. Specifically, the ability to utilize oxygen and burn fat at rest is assessed most accurately by analyzing the balance between oxygen and carbon dioxide in the breath, also known as the Respiratory Exchange Ratio. Studies have also proven this concept by showing that reduced fat oxidation at rest is a risk factor for diabetes even before the onset of elevated blood sugar levels during a fasted state (a condition also known as pre-diabetes).
Moreover, the likelihood of obesity can be accurately assessed by determining the metabolic level of an individual, in other words, whether one’s metabolism is faster or slower than expected based on age, gender, and body size. The gold standard for analyzing a person’s metabolism is also breath analysis.
Conclusion
Diabetes is undeniably a lifestyle disease that stems from physical inactivity and poor nutrition habits. Combined, they lead to impaired cellular oxygen uptake ability and fat accumulation, leading to insulin resistance. As a result, addressing diabetes through nutrition and exercise should be a priority for everyone looking to avoid it or overcome it.
An Ounce of Prevention - Hyperion Health Blog




